Sodium Thiosulfate Australian Registration
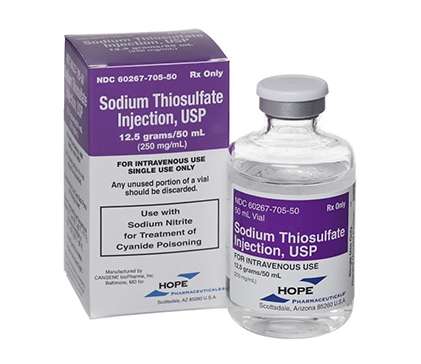
HL Pharma is the distributor of the ARTG registered product Hope Pharmaceuticals Sodium Thiosulfate Pentahydrate 12.5 g/50 mL solution for Injection Vial (the Product), sponsored by Hope Pharmaceuticals Pty Ltd (AUST R 276733)
As an ARTG-registered product, the Product satisfies the rigorous standards of the TGA along with pharmacovigilance requirements that unlicensed products from overseas may not provide.
We are aware that in the past, unlicensed sodium thiosulfate products sourced from overseas have been supplied in Australia under the Special Access Scheme (SAS) or Authorised Prescriber Scheme (APS).
We have informed the TGA that the Product, as an ARTG-registered good, is commercially available and able to be supplied to the market, such that the sourcing of unapproved products for supply under the SAS or APS is no longer appropriate.
The TGA has agreed, in our recent correspondence with them, that "the use of SAS and APS are generally not appropriate in circumstances where a registered medicine is clinically suitable for the proposed indication”. The TGA has also informed us that "the entry for sodium thiosulfate in [its] internal database of unapproved products contains a note alerting users to the existence of registered sodium thiosulfate goods. The note expressly states that consideration should be given to requesting further information from applicants as to the need for the medicine before making a decision on a request for a SAS approval or APS authority".
For your information, Sodium Thiosulfate Injection 12.5g/50ml Hope AU 50ml is stocked by CHS and Clifford Hallam (CH2), or can be purchased directly from HL Pharma.